Vascular access, widely used for hemodialysis and made available through surgically placed arteriovenous fistulas (AVF), are prone to failure because of stenosis and ultimately thrombosis. This contributes to significant morbidity, mortality and cost of care for patients with end-stage renal disease (ESRD). Multiple factors lead to failure of AVF in ESRD including endothelial cells getting activated in the veil wall, high levels of pro-coagulant factors in blood and stagnant blood flow in areas of low wall shear stress (WSS). To investigate these factors, we have teamed up with Dr. Mary Hammes, Department of Medicine, Nephrology Section, to develop a novel millifluidic device and flow platform to make patient-specific vein models that replicate thrombosis. We then use this 3D model to link blood conditions in renal failure with flow, using blood from ESRD patients, coagulation factors, and physical and biochemical modeling.
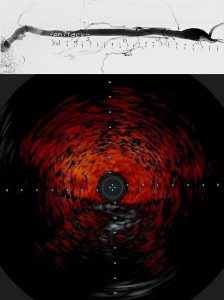
Specifically, we are building 3 dimensional vein models of the brachiocephalic arch (BCA) where thrombosis and stenosis is likely to occur. These models are reconstructed from venogram (above) and intravascular ultrasound (IVUS; below) imaging of the BCA in ESRD patients to capture the exact geometry and hence rheological conditions of blood flow. We are developing 3D modeling techniques using AutoCAD and Matlab for this purpose.
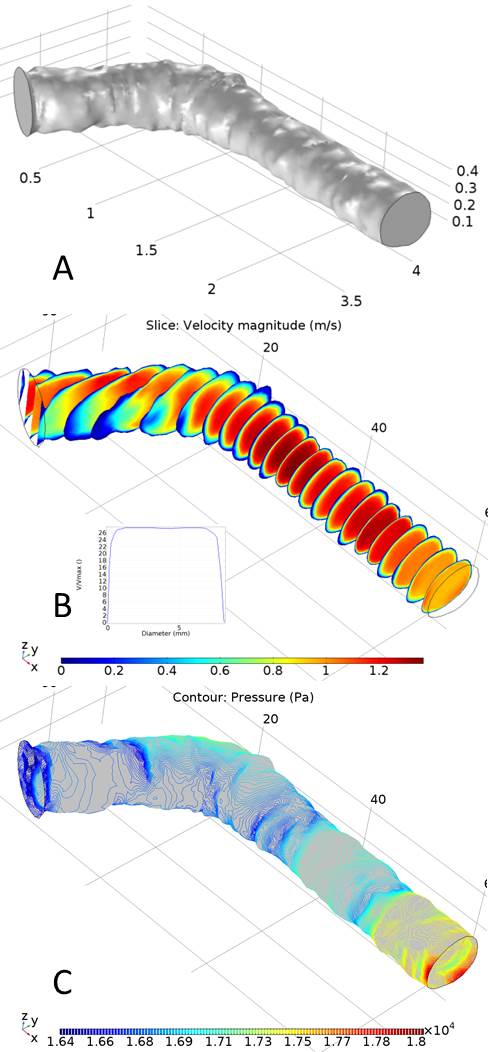
Fluid dynamics simulations using COMSOL Multiphysics allow us to estimate flow patterns in critical areas of the cephalic arch model, which we use to direct our flow and imaging experiments. Figure A on the right shows the 3D model of the cephalic arch created for a ESRD patient undergoing regular dialysis 12 months after the AVF placement. Figures B and C show the velocity and pressure profiles in the same same vein using blood flow velocity and pressure measurements on the patient, using Doppler.
The 3D model of the same AVF vein is then fabricated into a millifluidic device, using 3D printing. The fluidic device recapitulates the AVF vein in dimensions and geometry, both of which play critical roles in blood flow rheology (e.g., enhanced wall shear stress, high Reynolds number, vortex flow, etc.) and pathology.

We are perfusing the fluidic devices using blood or proxy fluid, under controlled flow conditions, including pulsatile flow, as seen in AVF vein. Unlike normal veins, which do not experience pulsatile flow of blood, AVF vein undergoes pulsed flow due to its proximity to the arterial connection via the AVF graft. We are using an advanced fluid flow setup from Elvesys that allows volumetric flow up to 1,000 mL/min and under continuous re-circulation. In-line pressure is continuously monitored using a pressure flow sensor that can be used to dynamically control blood flow rate in the fluidic device. Blood perfusion studies are performed on blood from ESRD patients and healthy controls under control and altered flow parameters, cellular elements and chemical factors in blood to stimulate thrombosis.
Future plans include endothelializing the fluidic devices to capture the role of the vein’s lumen in activating platelets and pro-coagulant factors in initiating thrombosis. Finally, we will track thrombosis and clot formation using live-cell, time-lapse microscopy and characterize thrombotic clots using physical and mechanical and biochemical assays.
This work is being conducted by Andres Moya-Rodriguez, as part of his thesis project, and in collaboration with a team of clinicians and physician-scientists including Dr. Mary Hammes, Nephrologist, Dr. Sandeep Nathan, Cardiologist and expert in IVUS imaging, and Dr. Luka Pocivavsek, Assistant Professor in Surgery. We are grateful to Ginny and Simon Aronson for their kind support for this project.