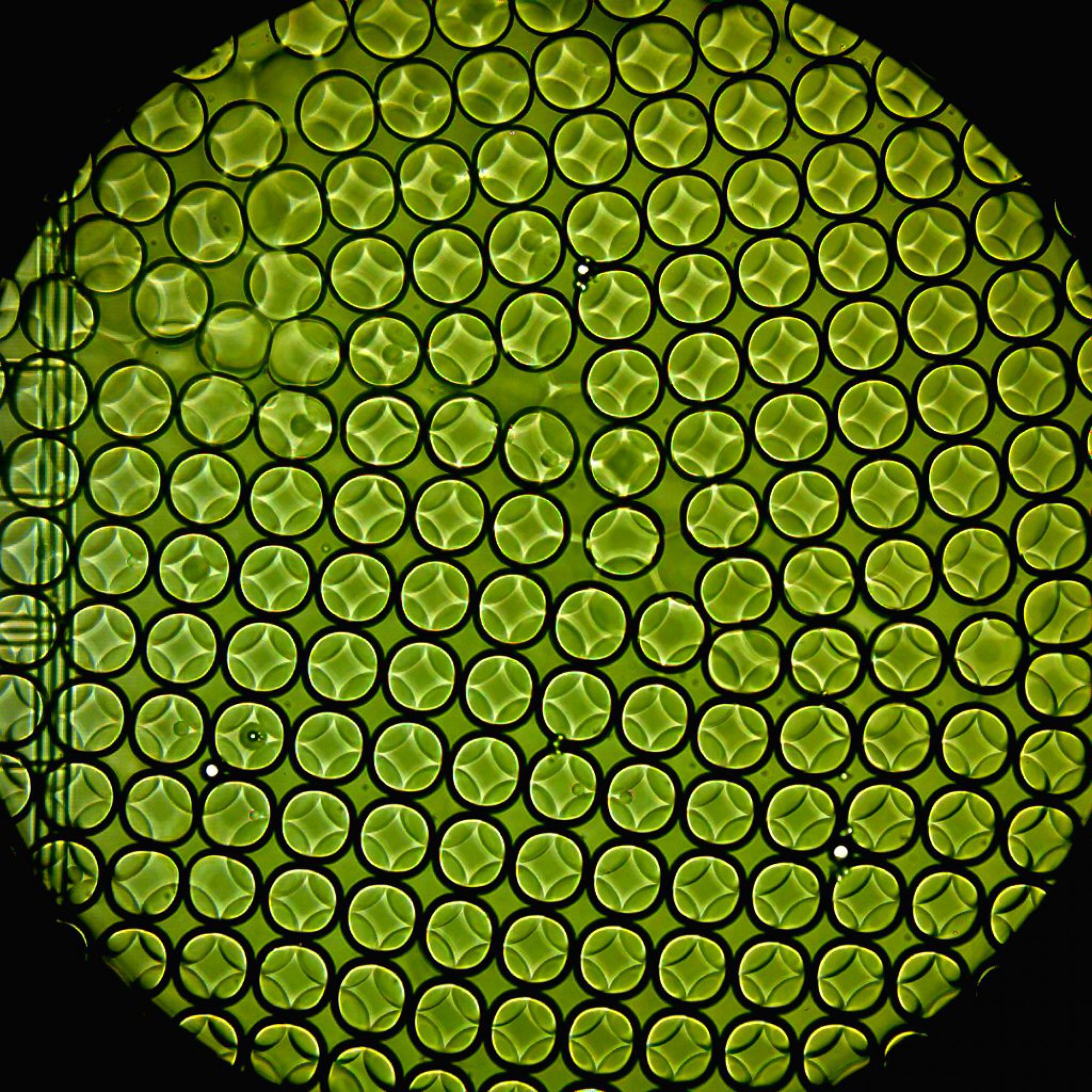
High-throughput drug screening conducted using various technologies, such as robotic handling and micro-titer plates, have significantly advanced drug development. However, the costs and time associated with such technologies are exorbitant. Droplet microfluidics provides a popular lab-on-a-chip technique where reagents may be combined in pico-liter volumes in a fast and controlled manner. We use such devices to generate water-in-oil emulsion droplets at high throughput (~5k-10k drops per second) that efficiently encapsulate cells in presence of drugs. Reducing the size of the reaction compartments to pico-liter volumes allow us to be parsimonious with reagents while the large number of droplets (~105-106) provides superior statistical resolution.
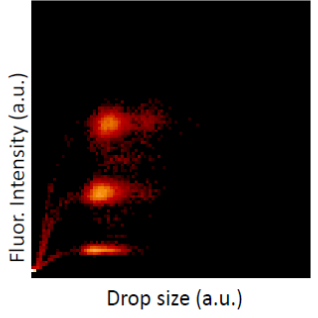
In this project, we use two sets of microfluidic devices to test the efficacy of cancer chemotherapy drugs on human cell lines where the drug concentrations are systematically varied. A fluorescent dye indicates the chemotherapy drug concentration (see figure to the left; drug concentration is proportional to the fluorescent intensity of the drop). The efficacy of the drugs is measured as functions of exposure time and drug concentration from the cell-fate of human lymphoblast cells (LCL) in each drop after co-incubation, using fluorescent live/dead cell-fate reporter kit.
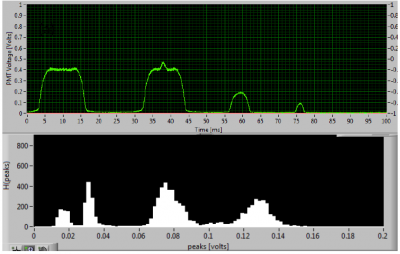
After the drops are generated, we collect the drops off chip, incubate them for different time intervals, and then re-inject the drops into a second microfluidic chip where they are excited by a laser beam and the resulting fluorescent emission from the drug and cells (dead/alive) are simultaneously detected using photo-multiplier tubes (PMT). The PMT voltage, indicative of fluorescent intensity, reports drug concentration, while the duration of the signal distinguishes a drug-laden drop from a live cell (bottom, left). Overall statistical information is aggregated onto a heat-map shown on bottom, right.